172x Filetype PDF File size 0.31 MB Source: sccollege.edu
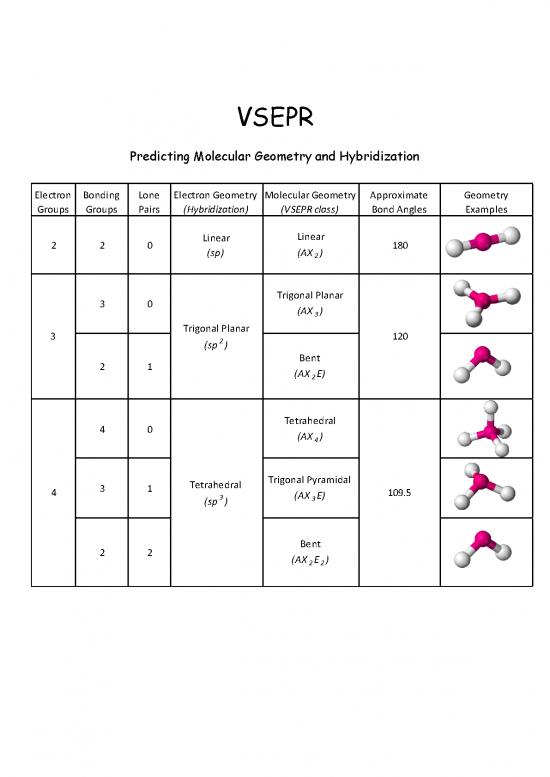
VSEPR
Predicting Molecular Geometry and Hybridization
Electron Bonding Lone Electron Geometry Molecular Geometry Approximate Geometry
Groups Groups Pairs (Hybridization) (VSEPR class) Bond Angles Examples
Linear
2 2 0 Linear 180
(sp) (AX2)
3 0 Trigonal Planar
(AX3)
3 Trigonal Planar 120
(sp2)
2 1 Bent
(AX2E)
Tetrahedral
4 0 (AX )
4
Trigonal Pyramidal
4 3 1 Tetrahedral (AX E) 109.5
(sp3) 3
2 2 Bent
(AX2E2)
Electron Bonding Lone Electron Geometry Molecular Geometry Approximate Geometry
Groups Groups Pairs (Hybridization) (VSEPR class) Bond Angles Examples
Trigonal Bipyramidal
5 0 (AX )
5
Seesaw 120 (in plane)
4 1 Trigonal (AX E) 90 (above and
4 below)
5 Bipyramidal
(sp3d)
3 2 T-Shaped
(AX3E2)
2 3 Linear 180
(AX2E3)
6 0 Octahedral
(AX6)
5 1 Square Pyrimidal
(AX5E)
6 4 2 Octahedral Square Planar 90
(sp3d2) (AX4E2)
3 3 T-Shaped
(AX3E3)
2 4 Linear
(AX2E4)
no reviews yet
Please Login to review.