177x Filetype PPTX File size 0.96 MB Source: anvari.net
Oxidation of Aldehydes
• Aldehydes oxidize readily to form carboxylic acids.
• Ketones do not undergo oxidation.
General, Organic, and Biological Chemistry: Structures of Life, 5/e © 2016 Pearson Education, Inc.
Karen C. Timberlake
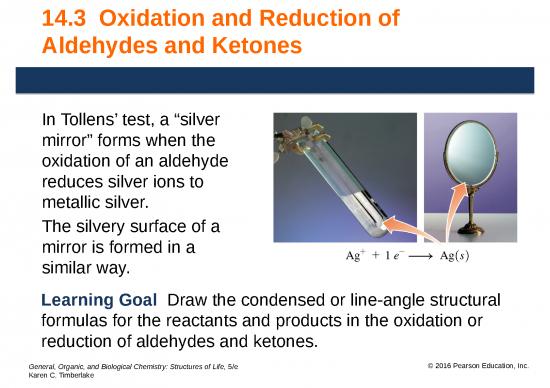
Tollens’ Test: Silver Mirror
Tollens’ test
• may be used to distinguish between aldehydes and
ketones.
+
• utilizes Tollens’ reagent, which is a solution of Ag (AgNO3)
and ammonia.
• oxidizes aldehydes but not ketones.
• reduces Ag+, as the aldehyde is oxidized and forms a layer
called a “silver mirror” on the inside of the container.
General, Organic, and Biological Chemistry: Structures of Life, 5/e © 2016 Pearson Education, Inc.
Karen C. Timberlake
Benedict’s Test
Benedict’s test
• gives a positive result with
compounds that have an
aldehyde functional group and an
adjacent hydroxyl group.
• utilizes Benedict’s solution, which
2+
contains Cu (CuSO4). When the
solution is added to this type of
aldehyde and heated, a brick-red
solid of Cu2O forms
• is negative with simple aldehydes
and ketones.
General, Organic, and Biological Chemistry: Structures of Life, 5/e © 2016 Pearson Education, Inc.
Karen C. Timberlake
Benedict’s Test: 2-Hydroxy Aldehyde
Because sugars such as glucose contain this type of aldehyde
grouping, Benedict’s reagent can be used to determine the
presence of glucose in blood or urine.
General, Organic, and Biological Chemistry: Structures of Life, 5/e © 2016 Pearson Education, Inc.
Karen C. Timberlake
Reduction of Aldehydes and Ketones
Aldehydes and ketones
• are reduced by hydrogen (H ) or sodium borohydride
2
(NaBH ) and a catalyst such as nickel, platinum, or
4
palladium.
• are reduced to alcohols by decreasing the number of
carbon–oxygen bonds.
General, Organic, and Biological Chemistry: Structures of Life, 5/e © 2016 Pearson Education, Inc.
Karen C. Timberlake
no reviews yet
Please Login to review.